By Alton Parrish.
At the suburban Chicago laboratory, a group of scientists has seemingly defied the laws of physics and found a way to apply pressure to make a material expand instead of compress/contract.
“It’s like squeezing a stone and forming a giant sponge,” said Karena Chapman, a chemist at the U.S. Department of Energy laboratory. “Materials are supposed to become denser and more compact under pressure. We are seeing the exact opposite. The pressure-treated material has half the density of the original state. This is counterintuitive to the laws of physics.”

Because this behavior seems impossible, Chapman and her colleagues spent several years testing and retesting the material until they believed the unbelievable and understood how the impossible could be possible. For every experiment, they got the same mind-bending results.
“The bonds in the material completely rearrange,” Chapman said. “This just blows my mind.”
This discovery will do more than rewrite the science text books; it could double the variety of porous framework materials available for manufacturing, health care and environmental sustainability.
Scientists use these framework materials, which have sponge-like holes in their structure, to trap, store and filter materials. The shape of the sponge-like holes makes them selectable for specific molecules, allowing their use as water filters, chemical sensors and compressible storage for carbon dioxide sequestration of hydrogen fuel cells. By tailoring release rates, scientists can adapt these frameworks to deliver drugs and initiate chemical reactions for the production of everything from plastics to foods.
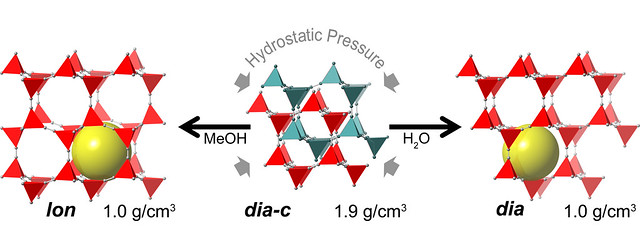
Pressure-induced transitions are associated with near 2-fold volume expansions. While an increase in volume with pressure is counterintuitive, the resulting new phases contain large fluid-filled pores, such that the combined solid + fluid volume is reduced and the inefficiencies in space filling by the interpenetrated parent phase are eliminated
Credit: ANL
“This could not only open up new materials to being porous, but it could also give us access to new structures for selectability and new release rates,” said Peter Chupas, an Argonne chemist who helped discover the new materials.
The team published the details of their work in the May 22 issue of the Journal of the American Chemical Society in an article titled “Exploiting High Pressures to Generate Porosity, Polymorphism, And Lattice Expansion in the Nonporous Molecular Framework Zn(CN)2 .”
The scientists put zinc cyanide, a material used in electroplating, in a diamond-anvil cell at the Advanced Photon Source (APS) at Argonne and applied high pressures of 0.9 to 1.8 gigapascals, or about 9,000 to 18,000 times the pressure of the atmosphere at sea level. This high pressure is within the range affordably reproducible by industry for bulk storage systems. By using different fluids around the material as it was squeezed, the scientists were able to create five new phases of material, two of which retained their new porous ability at normal pressure.
“By applying pressure, we were able to transform a normally dense, nonporous material into a range of new porous materials that can hold twice as much stuff,” Chapman said. “This counterintuitive discovery will likely double the amount of available porous framework materials, which will greatly expand their use in pharmaceutical delivery, sequestration, material separation and catalysis.”
The scientists will continue to test the new technique on other materials.
The research is funded by the U.S. Department of Energy’s Office of Science.
Contacts and sources:
Tona Kunz
DOE/Argonne National Laboratory


